
Although VSEPR theory predicts the distribution of the electrons, we have to take in consideration of the actual determinant of the molecular shape. Thus, the molecule's shape reflects its equilibrium state in which it has the lowest possible energy in the system. The electrons and the nuclei settle into positions that minimize repulsion and maximize attraction. The shape of a molecule is determined by the location of the nuclei and its electrons. Using the VSEPR theory, the electron bond pairs and lone pairs on the center atom will help us predict the shape of a molecule. An electron group can be an electron pair, a lone pair, a single unpaired electron, a double bond or a triple bond on the center atom. VSEPR focuses not only on electron pairs, but it also focus on electron groups as a whole. Thus, electron pairs will spread themselves as far from each other as possible to minimize repulsion. The valence-shell electron-pair repulsion (VSEPR) theory states that electron pairs repel each other whether or not they are in bond pairs or in lone pairs. Now that we have a background in the Lewis electron dot structure we can use it to locate the the valence electrons of the center atom. Valence-Shell Electron-Pair Repulsion Theory The presence of lone pair causes slight distortion from 109☂8’ to 107☄8’.\) This shape may either be described as tetrahedral or pyramidal. These four pairs of electrons give rise to a tetrahedral structure where three positions are occupied by H atoms and fourth position by the lone pair. The outer shell then has a share in eight electrons, that is, three pairs bonded and one lone pair.

Three electrons of N are bonded with hydrogen and the rest two which do not take part in bonding form the lone pair. Nitrogen is a group 15 element and therefore has 5 electrons in its outmost shell. Q: On the basis of VSEPR theory explain the structure of NH 3 molecule.Īns: In ammonia, N is the central atom. Quantum mechanics and atomic orbitals can give more sophisticated predictions when VSEPR is inadequate. VSEPR also predicts that group-2 halides such as will be linear when they are actually bent.For example, VSEPR predicts that and will have the same bond angles, but structural studies have shown the bonds in the two molecules are different by 12 degrees. First, the idealized bond angles do not always match the measured values.VSEPR is simple and useful but does not work for all chemical species.Rather, it is an algorithm that accurately predicts the structures of a large number of compounds. It does not explain or attempt to explain any observations or predictions.
#VSEPR NOTATION DOWNLOAD#
You can download VSEPR Theory Cheat Sheet by clicking on the download button below If there are no unpaired electrons in the compound being assessed, the molecular and electron pair geometries will be the same. The main difference between molecular geometry and electron pair geometry is that molecular geometry does not include unpaired electrons, whereas electron pair geometry includes both bonded atoms and unpaired electrons. Electron Pair Geometry: This is the 3-D arrangement of electron pairs around the central atom of a polyatomic ion or molecule.
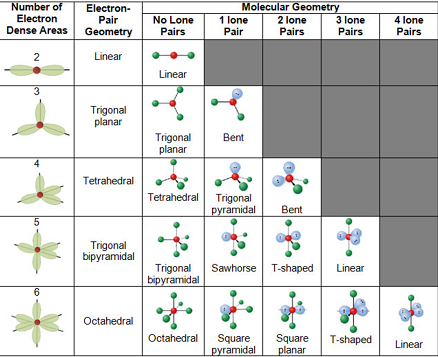
Molecular Geometry: This is the 3-D arrangement of bonded atoms in a polyatomic ion or molecule.Lone Pair: This refers to a pair of valence electrons that are not shared with another atom.Bond Angle: This is the angle between a bonded atom, the central atom, and another bonded atom.The following terms are commonly used in discussing the shapes of molecules. Each shape has a name and an idealized bond angle associated with it. It can predict the shape of nearly all compounds that have a central atom, as long as the central atom is not a metal.VSEPR models are based on the concept that electrons around a central atom will configure themselves to minimize repulsion, and that dictates the geometry of the molecule.The VSEPR model predicts the 3-D shape of molecules and ions but is ineffective in providing any specific information regarding the bond length or the bond itself.



 0 kommentar(er)
0 kommentar(er)
